Important dynamics in the carbon cycle


Carbon can be stored in the atmosphere, ocean, soil, vegetation and in the lithosphere. The carbon cycle is a closed system. To limit temperature increase also the amount of carbon stored in the atmosphere needs to be limited. Limiting the amount of carbon stored in the atmosphere means that it needs to be stored in one of the other sinks and this will have an impact on those sinks.
Introduction
Climate policy aims mainly to reduce CO2 in the atmosphere because a higher concentration of CO2 in the atmosphere is leading to higher global mean temperatures. This is because CO2 and other greenhouse gasses trap the infrared heat coming from earth. In this piece we explain where the carbon is stored and how the flows are (carbon cycle). This is important because the dynamics of the carbon cycle are behind the mitigating strategies and technologies that are put in place to reach net zero by 2050. We will focus on these technologies with link to the carbon cycle in more detail in our coming publications. We end with a conclusion.
Carbon cycle
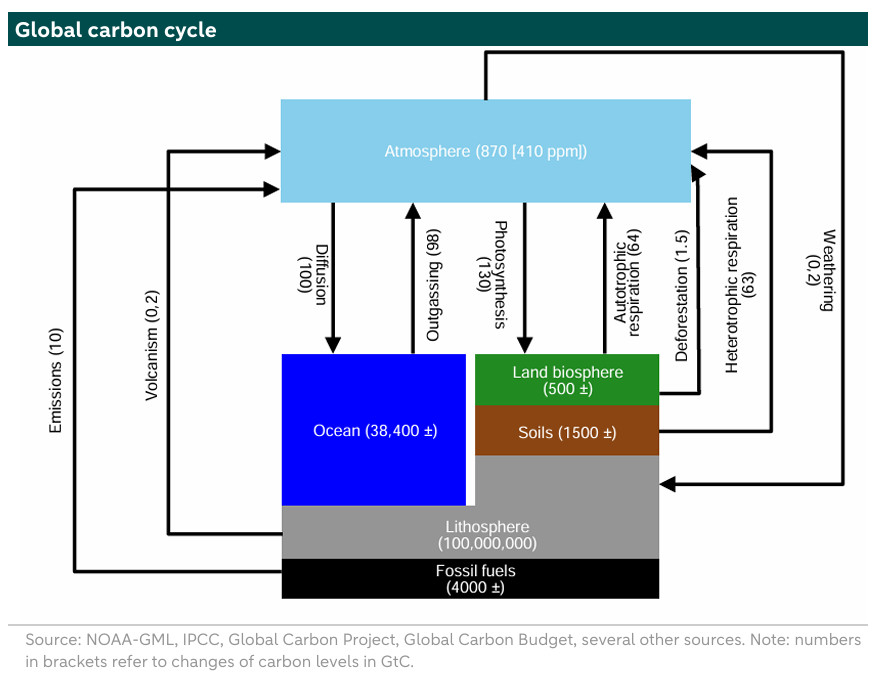
The carbon cycle is a closed system where carbon is stored and how the flows are. Carbon can be stored in the lithosphere, which is the crust and uppermost solid mantle (including fossil fuel reserves), in the soil, in plants, in the ocean and in the atmosphere. The diagram on the next page (from 2018) shows where the carbon is stored and their changes (in brackets). For example, the atmosphere stores 870 gigaton of carbon, which is equivalent to 410 parts per million of CO2 volume. In climate policy we mainly focus on the CO2 concentration in the atmosphere because its higher concentration leads to higher global mean temperatures. So the atmosphere is a sink of carbon stored in CO2 gas molecules. Besides the atmosphere, carbon can be stored in the lithosphere, the ocean and the soil. The largest sink on earth is the lithosphere, where 100,000,000 Gt of carbon is stored. This is followed by the ocean, which stores 38,400 Gt of carbon. If all the carbon currently stored in the ocean would be in the atmosphere this would lead to a rise in temperatures of 8 to 25°C. Finally, in the soil, 1,500 Gt of carbon is stored. All the numbers are in gigaton of carbon or GtC. This is not the same as Gt CO2. 1 GtC equals 3.66 Gt CO2.
There is a large interaction between the atmosphere and the ocean. The ocean absorbs extra carbon through diffusion, and releases it through outgassing, resulting in a net CO2 uptake (100 GtC-98 GtC). Similarly, the biosphere (plants) is also a net uptaker of CO2 (130-64 GtC-1.5 GtC). Meanwhile, decompositions of soil organic matter and plant litter by soil microbes result in emissions of CO2 and other fossil fuels (heterotrophic respiration 63 GtC and 10 GtC, respectively).
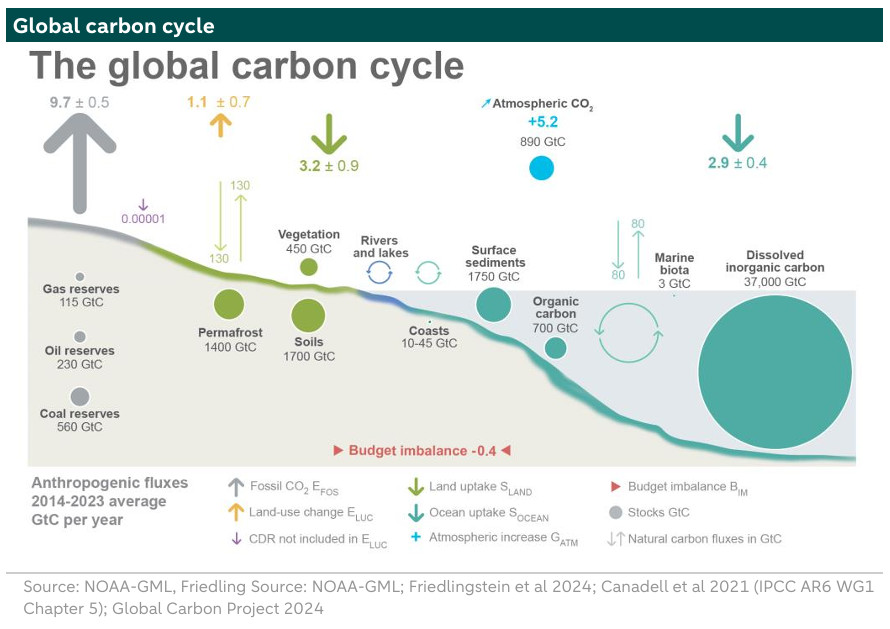
The graph below is a different representation of the carbon cycle. The numbers are the global averages for 2014-2023 in GtC. The circles represent the sinks or where the carbon is stored and the arrows the annual flows in GtC. In the below, the ocean is the largest sink with 37,000 GtC, as it excludes references to the lithosphere (which is actually the largest sink) - although oil, coal and gas reserves are included. Moreover, the below includes details on the amount of carbon stored in the permafrost, namely 1,400 GtC.
Carbon stored in the atmosphere
When energy from the Sun reaches the Earth part of this is reflected by the Earth (albedo) and part of this is absorbed by the earth. The Earth radiates this energy back as heat (infrared). This infrared radiation travels to the atmosphere where it is eventually absorbed by CO2 molecules and other greenhouse gas molecules. The molecules vibrate and re-emit the energy back in all directions. About half of this goes to space and about half of it returns to Earth as heat. As more carbon ends up in the atmosphere there are also more CO2 molecules in the atmosphere that absorb and re-emit this infrared radiation, resulting in higher global mean temperatures. Ultimately, this means that the concentration of CO2 in the atmosphere is controlling the climate on Earth. Currently the CO2 concentration in the atmosphere 427 ppm (16 March 2025) or 908 GtC.
Carbon stored in the ocean
What happens if the CO2 is not in the atmosphere but taken up by the ocean? Oceans take up 25% of the CO2 emissions each year so this mitigates the impact of CO2 in the atmosphere. Half of the emissions stay in the atmosphere. Gases like CO2 dissolve into seawater. CO2 dissolves better in colder water than in warmer water. The CO2 gas turns into CO2 aqueous form, which then dissolves. CO2 reacts with water to form bicarbonate and carbonate. This lowers the CO2 in the ocean and therefore, the atmosphere adds CO2 to the ocean again (more on this below). This process in the ocean is the reason why oceans take up so much CO2.
There are two equilibriums that one needs to keep in mind. To start, there is the equilibrium between CO2, bicarbonate and carbonate in the seawater. The ratio for seawater with pH of 8 is 1:88:11 for CO2: bicarbonate: carbonate. The acidity in the ocean can disrupt this equilibrium. In general, in seawater with low pH (more acid) there is more CO2 in aqueous form than in seawater with higher pH. More acid seawater has a negative impact on for example coral reefs. So an equilibrium with lower CO2 aqueous form is preferred.
Another important equilibrium is the CO2 concentration in the seawater compared to the atmosphere. If one or both are increasing this results into CO2 flowing from the sink with the highest concentration to the one with the lowest. For example, if the concentration of CO2 in the seawater is lower that in the atmosphere, then CO2 will move from the atmosphere to the ocean. If we are successful in reducing CO2 in the atmosphere, the concentration of CO2 in atmosphere will be lower than the one in seawater. As a result, CO2 will also move from ocean to the atmosphere within 1 year. This an important dynamic to understand.
How does water temperature influence CO2 concentration in ocean? CO2 and other gases are less soluble in warmer seawater as higher temperatures leave less space for CO2. This will result in the ocean releasing CO2 to the atmosphere. This is one of the reasons why seawater around the equator naturally releases CO2 to the atmosphere.So overall, oceans have much more capacity to store carbon and CO2 than the atmosphere, but there are specific dynamics that influence this storage capacity such as temperature, concentration of CO2 in the atmosphere and acidity.
Carbon stored in the terrestrial sphere
Next to the atmosphere and the ocean, carbon can also be stored in living plants and in soils. The terrestrial carbon stocks include forests, soils, wetlands, permafrost and other land-based ecosystems. Plants use CO2 from the atmosphere, water from the soil and energy from the Sun to produce glucose and oxygen, the latter being a byproduct. Next, plants convert the glucose back into energy, a process which requires oxygen again. Half of the CO2 plants take from the atmosphere for photosynthesis is returned to the atmosphere due to plant maintenance (making leaves and stems) in the form of respiration. How active plants are depends on the amount of sunlight, moisture in the soil, CO2 concentration in the atmosphere, nutrients in the soil and temperatures. In general, the more of these aforementioned elements, the better, with the exception being very high temperatures and very wet conditions. If there are more plants and/or plants are more active, more CO2 is taken out of the atmosphere. However, the size of carbon storage depends also on whether plants invest in more durable structures, such as stems, which are more difficult the break down when the plant dies. Higher temperature and more moisture are the ideal environment to break down plant material (that is, there is a high turnover rate). In the tropics the amount of living biomass is high because of a high degree of photosynthesis and high decomposition rate.
Next to plants, soils are also an important carbon sink. In the soil, microbes break down organic matter and by doing so, release CO2 in the form of respiration. Stems take longer to break down than leaves. The largest amounts of dead biomass underground are found in high latitudes where photosynthesis, temperature, moisture and decomposition rates are low. Wetlands and permafrost regions also have a lot of carbon stored. If wetlands are drained, then the soils become exposed to oxygen and CO2 is released. In the case of the permafrost thawing, microbes will get a lot of nutrients and with the availability of oxygen these soils are decomposed resulting in the release of CO2.
Carbon stored in the lithosphere
The last place to store carbon is the lithosphere. This is the largest sink of carbon. The lithosphere is the solid, outer part of the Earth. Carbon storage in the lithosphere is on a much longer timescale than storage in the terrestrial. In the lithosphere carbon is stored in sediments and rocks. Fossil fuel deposits are also part of the lithosphere. Fossil fuels are formed because of geological processes acted on the remains of ancient photosynthesis. Coal is originally from carbon stored in the terrestrial land, while oil and gas are originally from carbon stored in the ocean (sinking of ocean biomass). If fossil fuels are taken out of the lithosphere to be burnt, this releases carbon stored from the lithosphere to the atmosphere. Low (or lower) carbon emission technologies such as electric vehicles, alternative low emission fuels and solar try to make us less dependent on burning fossil fuels.
On the other hand, rock weathering is a way to store carbon in the lithosphere. CO2 from the atmosphere reacts with water to form carbonic acid, which then reacts with certain types of rocks, such as sedimentary rock/carbonate rocks (limestone), volcanic rock (basalt), magnesium rich rock (serpentine) or magnesium rich and iron rich rock (olivine). This reaction implies that the carbonic acid dissolves the rock. More rock weathering means less CO2 in the atmosphere. There are carbon sequestration techniques that enhance the natural way of rock weathering so that more carbon can be stored in the lithosphere ().
Conclusion
There are lot of ways to store carbon. Carbon can be stored in the atmosphere in the form of CO2 gas molecules. But an increasing amount of carbon in the atmosphere results in higher global mean temperatures, which is something we would like to avoid. The alternative is carbon storing elsewhere, such as in vegetation, soils, oceans and the lithosphere. There are several ways to get carbon from the atmosphere stored into another sphere, such as planting trees, carbon capture and storage, enhanced rock weathering and rewetting wetlands. While carbon, storing is important, an often overlooked aspect which is equally as important is avoiding that sinks release carbon to the atmosphere. One way to reduce the CO2 emissions is to replace fossil fuels into cleaner energy sources. Other ways involve keeping the permafrost frozen, to avoid draining wetlands, and keeping the carbon stored in the ocean (so avoid warming of the oceans). We want to make sure that sinks remain a sink and the flows to the atmosphere are reduced.