SustainaWeekly - Shinning a light on battery technologies

In a world where renewable power becomes dominant, a way to store energy is crucial. In this week’s SustainaWeekly we shine the spotlight on battery technologies, not only existing ones, but also emerging technologies. We focus on the pros and cons of the various technologies. In a separate note, we go on to look at the issue of ESG data disclosures from the issuers of sustainable covered bonds. In our final note, we look at the issuance of sustainability-linked bonds so far this year, as well as recent demand and pricing dynamics.
Economist:
The problem with renewables is intermittency and batteries can solve this problem. However, batteries also have their challenges: energy density, life cycles, use of materials. The choice of technology is mainly a trade-off between energy density, life cycles and availability and costs of materials with safety always being at a high standard. Emerging battery technologies could solve some of the challenges that the current technologies face. Policy & Regulation:
The market for sustainable covered bonds has been growing steadily. However, still much improvement is needed with regards to ESG data disclosure. The ECBC’s Harmonised Transparency Template (HTT) already offers issuers the opportunity to disclose the most important ESG data but not many issuers do so. Next year, ESG data reporting in HTT will be expanded and will become mandatory for sustainable covered bond issuers.Strategist:
Stronger demand for SLBs versus regular or green bonds is not translating into lower new issue concessions. Indeed, last week’s SLB issuance by Wienerberger came with a significant pick-up to other building material names and high curve steepness. Yet the limited specification by this issuer on how exactly it will reduce emissions and an absence of absolute emission intensity disclosures could explain the premium it had to pay.
The pros and cons of battery technologies
The problem with renewables is intermittency but batteries can solve this problem
Batteries store energy for later use, for mobility and portable dices
Batteries also have their challenges: energy density, life cycles, use of materials
The choice of technology is mainly a trade-off between energy density, life cycles and availability and costs of materials with safety always being at a high standard
Emerging battery technologies could solve some of the challenges that the current technologies face
To reach net-zero by 2050 renewables play a crucial role. The main problem with renewables is intermittency, so they come and go and patterns can be difficult to predict. Therefore, we need a way to store this energy. There are two main objectives/reasons to store energy: storage for home/office or for mobility. An example of the former is a home battery that stores energy produced during the day by solar panels to consume it when the sun sets. So this battery is used to store energy from renewable sources to use it at a later moment. An example of the latter is a battery used in an electric car instead of an internal combustion engine that burns fossil fuels. So this is a battery to store energy to drive instead of burning fossil fuels and thereby reducing greenhouse gas emissions. Batteries are crucial in the energy transition. However, there are quite some challenges. Batteries for home storage are expensive, generally not supported by subsidies and they rely on materials that are in short supply. Batteries used in mobility need higher energy density to increase the range. They are also expensive because of the critical metals used, while these metals could also be in a shortage in the future. In this report we focus on the current and emerging battery technologies to solve some of these challenges. We start with some basics on batteries, then look at the batteries that are currently available followed by the emerging technologies.What is a battery?A battery is a device that produces electricity from a chemical reaction. Each battery has a cell that has three components: two electrodes (positive and negative) and an electrolyte between them. The electrodes are immersed in chemicals inside the battery. The chemicals react with the metals causing excess electrons to build up on the negative electrode producing a shortage of electrons on the positive electrode. If you connect a circuit then there is a way for the electrons to travel from the negative to the positive side. So conventional batteries store energy by using chemical reactions to trap ions that move from one electrode to the other. As noted above, batteries are used to store renewable energy for later use and for electric vehicles. There are different types of batteries. There are non-rechargeable batteries (alkaline, coin cell) and rechargeable batteries (lead-acid, nickel-cadmium, nickel-metal hybrid and lithium-ion). They are explained in detail in the table below. Today, more than 50% of the entire market uses lithium-ion. These batteries are preferred in electric vehicles due to their high energy per unit mass, high power to weight ratio, energy efficiency and low self-discharge (source: ). The two most common concepts associated with batteries are energy density and power density. Energy density is measured in watt-hours per kilogram (Wh/kg) and is the amount of energy the battery can store with respect to its mass. Power density is measured in watts per kilogram (W/kg) and is the amount of power that can be generated by the battery with respect to its mass (source: ). So it is the rate of charging/discharging the battery. Other important aspects are thermal runaway especially for lithium-ion batteries and cycle life. Thermal runaway is defined as a critical condition arising during constant voltage charging in which the current and the temperature of the battery produces a cumulative, mutually reinforcing effect which further increases them, and may lead to the destruction of the battery. A battery cycle life is the number of charge, discharge or rest cycles a cell or battery can provide. Below we go into more details about lithium-ion batteries because they are currently the most used batteries Lithium-ion batteryAs indicated above, a lithium-ion battery is a family of rechargeable battery types. The battery has a cathode, anode, separator, electrolyte and two current collectors (positive and negative). The anode and cathode store the lithium. The electrolyte carries positively charged lithium ions from the anode to the cathode and vice versa through the separator. The movement of the lithium ions creates free electrons in the anode which creates a charge at the positive current collector. The electrical current then flows from the current collector through a device being powered (mobile phone, computer, EV) to the negative current collector. The separator blocks the flow of electrons inside the battery. While the battery is discharging and providing an electric current, the anode releases lithium ions to the cathode, generating a flow of electrons from one side to the other. When plugging in the device, the opposite happens: Lithium ions are released by the cathode and received by the anode (source: www.energy.gov). A battery can be packed in various ways: cylindrical, prismatic or pouch. Every way of packing has a different system of thermal management.

The battery's capacity and voltage are determined based on what type of active material is used in the cathode. Most cathodes contain nickel, lithium, cobalt and manganese but in different quantities. Some batteries contain aluminium. Each battery chemistry has a specific energy density, stability, safety and durability and one chemistry could be better suited for storage for electric vehicles and vice versa. Nickel has high capacity, manganese and cobalt have high safety and aluminium increases the power of the battery. The different types of lithium-ion batteries are mentioned in the table below. A higher energy density means that vehicles can travel further without the need to recharge. Safety and durability are also important to take into account. The anode is responsible for the lifespan and is mostly made of natural or synthetic graphite. The electrolyte and separator determine the safety of a battery.
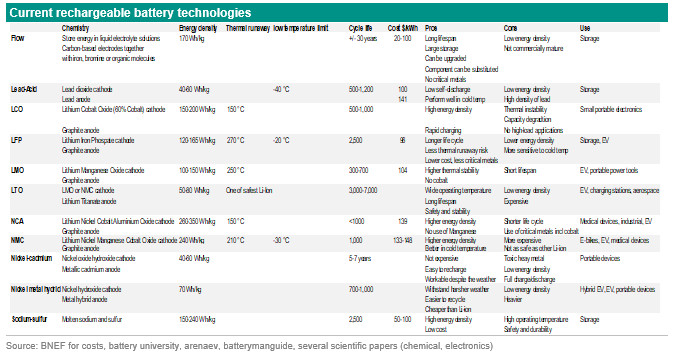
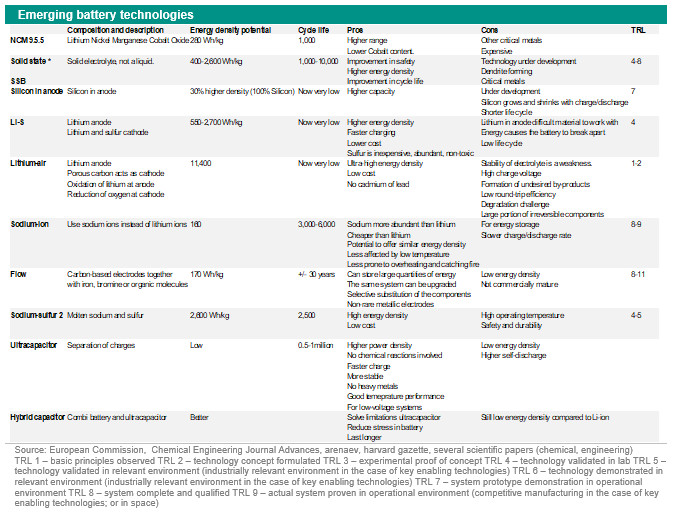
There are several ways to increase the energy density of a lithium-ion battery. First use more lithium, cobalt and other components. Second, change the composition of the battery and the materials used (battery chemistry). So, for example a lower use of cobalt and a higher use of nickel. A Nickel Cobalt Aluminium Oxide (NCA) battery has the greatest energy density. A Nickel Manganese Cobalt Oxide (NMC) battery has a lower energy than NCA battery but has a longer life cycle.
They both use critical metals and have relatively low thermal runaway temperatures (150-210 dergrees Celsius). A Lithium Iron Phosphate (LFP) battery offers thermal stability at even high temperatures, is low cost, has high cycles and high durability. A Lithium Titanate battery has low energy density and is expensive, but is one of the safest Lithium-ion batteries and has long life cycles. There is a drive to increase the range (higher energy density) especially for road transport, to increase the cycles, to reduce the number of critical metals used, and to reduce the costs in order to make batteries for storage and electric vehicles more affordable. One way to reduce costs and use of critical metals is to use another battery or change the battery chemistry. More car manufacturers are opting for an LFP battery instead of NMC. This is a cheaper, safer, has a longer lifespan and uses less critical metals but the energy density of LFP batteries is much lower than NMC batteries (see table above). LFP also seem to suffer from poor performance at very low temperatures. Another way to solve, or partly solve, the current challenges is waiting for the emerging battery technologies. More on this in the subsequent section. Emerging battery technologiesThe table above is an overview of the emerging battery technologies. Some of these are revamped editions of already available technologies such as NMC955, flow batteries, ultracapacitor and hybrid capacitor, some are already existing ideas but not rolled out on a commercial scale and others are new technologies. In terms of energy density, the battery technologies Lithium-Sulfur, Lithium-Air and Sodium-Sulfur (revamped) are very promising. But they have considerable stability, durability and/or safety issues. These challenges need to be solved first before they become available on a commercial scale. The technical readiness level of these technologies are between 1 and 5 depending on the technology. (the table below is an explanation of technical readiness levels